Amped Up
Amped Up

In 2018, when Nirav Patel (’20) enrolled in UCLA Anderson’s Executive MBA program, the engineer had a dream: to produce a low-cost ventilator for hospital use.
Patel found an enthusiastic audience among fellow Anderson EMBA students Shannon Demus (’20), Aanchal Kumar (’20), Stephan Hwang (’20) and Vikram Saurabh (’20). They recruited another classmate, Dr. Dezireh Sevanesian (’20), a dentist who had completed a residency in clinical anesthesiology, and started planning fundraising efforts for a San Diego-based company called Amptron Medical.
Their mission: Save more lives.
Fast forward to spring 2020. The pandemic caused by COVID-19 has resulted in at least 100,000 deaths in the U.S. and disrupted the global economy. “Ventilators” is a trending buzzword as companies like General Motors, Ford, General Electric and Virgin pivot to produce the devices.
Patel and Sevanesian explain how their approach to ventilators is so different.
Nirav Patel: Five of us were on a team in the business plan development class taught by Professor Jeff Scheinrock. The purpose of the class was to come up with a project and write a business plan. After I introduced the idea, the team was really interested to learn about the medical-device domain. So, we decided to propose it to the Business Creation Option program.
Being EMBAs, we’re in a different phase of life than regular, full-time MBAs because everyone has a full-time job, some of us have families and young kids. We found that we worked really well together as a team. Everyone has tried to manage their priorities and helped support this project.
Dezireh Sevanesian: I was the last piece added to this group. I came in because three members on Nirav’s team could vouch for my work ethic after I’d worked with them on other projects through Anderson. My clinical expertise rounded out the depth and breadth of knowledge that the group had.
NP: I worked ten-plus years as an engineer in the respiratory-care product domain, including for a few of the major ventilator manufacturers, and I have held several patents. I’ve researched, designed and developed a broad spectrum of respiratory products, from critical care ventilators to home use ventilators to sleep apnea devices.
The underlying theory behind the product is that not every type of respiratory distress syndrome requires the most sophisticated and most expensive form of ventilator. Approximately 60 to 80 percent of the cases could be treated using just a simple, low-cost device.
During the BCO process, we talked to more than 30 clinicians and respiratory therapists throughout the United States and the world to validate our hypothesis: Was there a critical need for a low-acuity, low-feature ventilator? Everyone we talked to said, “Yes, there’s a need for this kind of product.”
DS: With Nirav at the helm, the team identified that access to ventilators was a pressing issue on a global scale. Last July, in their pitch-deck, they concluded that if a respiratory pandemic were to strike, at the very least it would strain the system by an additional 30 percent. This was before COVID-19 was even on the radar.
NP: We were in the very early stages of this project. We were still validating our hypothesis, and we were beginning to secure funding.
NP: We learned that hospitals were pooling and repurposing their existing ventilators for the COVID-19 surge that was being anticipated. That created a vacuum for ventilators to serve other distressed patients, like prematurely born children in the neonatal wards who need respiratory support.
DS: As soon as “ventilator” became a buzzword, everyone became a ventilator inventor. Amateurs in their garages were trying to make ventilators. But ventilators are extremely sophisticated life support devices. Even big players in industry are having trouble scaling up to meet the demand because of the scarcity of parts and because there’s been such a major supply chain disruption.
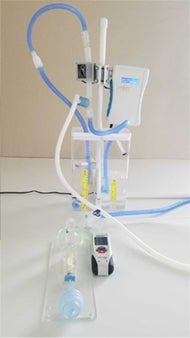
Amptron Simpli-Vent Prototype 2
DS: We decided that it was easier to build a device to treat patients within the neonatology ICU units, and help save the lives of pre-term and newborn children who are among our most vulnerable patients. That helps free up existing ventilators that can be used for COVID-19 patients.
In effect, we’re building something that works on the COVID-19 situation indirectly. That way, we’re not directly competing head-to-head with other companies trying to ramp up and make ventilators. All the things that are causing problems for the big players coming into the field — like the supply chain issue — we’re able to circumvent with our product.
NP: Our product, Simpli-Vent, is a “bridge” ventilator. It’s based on an existing mode of therapy that is quite commonly used in neonatal ICUs and extends that mode of therapy to support higher acuity patients. Our product is safe and is built with medical-grade components. We also have a provisional patent on the design of this product.
In the long-term, post COVID, our product can very well serve a niche neonatal and pediatric respiratory market. This market has very few competitors and lacks innovation. World Health Organization (WHO) estimates that over one million neonates die each year due to respiratory causes. What we have learned during our BCO market research is that in some developing countries, if a neonate cannot recover from respiratory illness on bubble CPAP (a noninvasive ventilation strategy for newborns), they do not survive. The hospitals there cannot afford critical care ventilators. Lack of access to respiratory support contributes to the large infant mortality rates in developing countries. Our product can serve such markets and save thousands of lives. That is the true value of our product and that is how we are different.
NP: Right now, we have one prototype device being evaluated at a hospital. We’re planning to build 50 of these prototype units for clinical evaluation. We want to raise $100,000 in grant money for that purpose.
DS: We’re applying for grants from the National Institutes of Health and from FEMA, and we’re exploring grant opportunities through local hospitals. We’ve also started discussions on the FDA’s Emergency Use Authorization process. As we move forward, we’re hoping to secure manufacturing contracts for the product and the accessories.
NP: We’ve reached out to UNICEF because this product, regardless of the COVID-19 situation, can save the lives of children and, specifically, children in emerging countries. The simplicity, the low cost and the ease of use are something that a lot of emerging, developing countries are looking for. This product will do very well there.
NP: We’re targeting the price point around $995, which is quite low compared to a traditional ventilator, which can cost anywhere from $25,000 to $35,000 per unit.
DS: As EMBAs, we’re set up to function this way. We’re used to coming on campus once a month and working remotely. I don’t feel like there’s been much difference in how we would’ve approached this prior to the pandemic, except that we’re missing out on the face-to-face interactions with classmates that we would’ve normally had. We’ve made up for that with a lot of virtual calls. Having said that, our success is entirely attributable to the team we have. Each member is vital to achieving our goals.
NP: In this situation where we’re all under lockdown, the collaboration has still been the same. We’ve gotten great support from the Anderson community. I’ve reached out to several faculty members — Jeff Scheinrock, George Abe, Dan Nathanson (who’s our advisor) — with text messages and Zoom calls, and they’ve been really responsive. Our advisor has stepped out of his normal routine and helped us at any hour of the day.
Also, for our secondary and market research, we have remote access to all the library resources at UCLA. We had a session with UCLA Anderson librarian Monica Hagan and she walked us through all the details. That’s been a great resource. Anderson has provided us with all the resources and connections to make this happen.